TCCV2 - Textile Competence Center Vorarlberg 2
Programme: COMET – Competence Centers for Excellent Technologies
Programme line: COMET-Project
Type of project: Strategic project 3.2 Solutions toward textile circularity
A solvent blend was developed for the selective dissolution and recovery of elastane for closed-loop recycling of textile waste
The textile industry faces significant challenges in recycling of fibres from clothes due to the complex blends of natural and synthetic polymers that are used in their construction. The recovery and reuse of fibre materials from old textiles into new, “fibre-to-fibre” recycling, requires that disparate components be separated with a high degree of purity. Only very few such closed-loop recycling technologies exist at the present time. One of the significant challenges is the presence of elastane, that is added in textile constructions to provide stretch and comfort (see figure 1). They are generally present in small proportions, in the range of about 1-5% *, but even in such low amounts they interfere in recycling of the other polymer types in clothing.
Thus, the elastane needs to be removed from textile blends to improve fiber recycling rates. The available options are either to degrade the elastane with heat, but that may damage other components in the blends or leave undesirable residues, or to selectively dissolve it in solvents. The typical solvents for elastane, dimethylformamide (DMF) and dimethylacetamide (DMAc), are toxic and not environment friendly, which makes it difficult to handle them in large quantities.
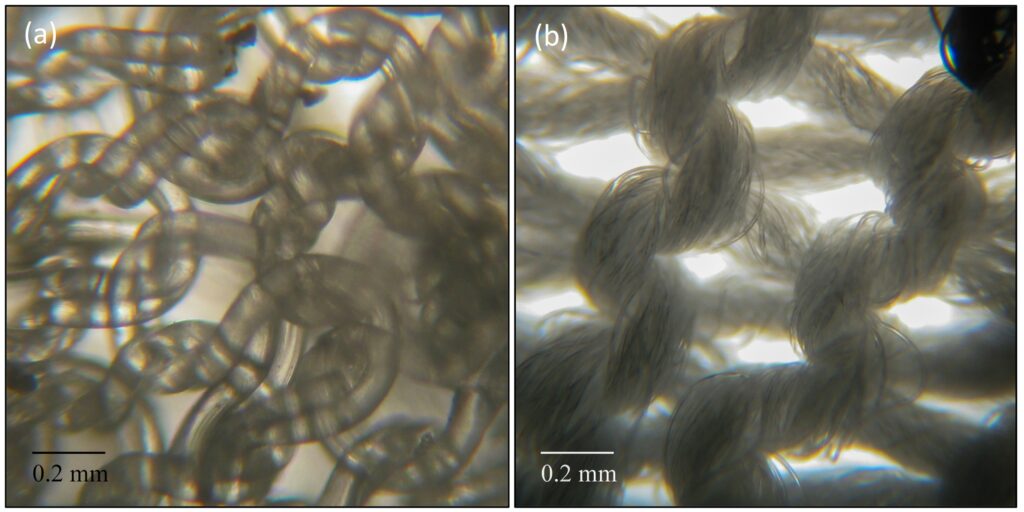
Figure 1: Elastane core structure (a) usually covered with another fibre type like polyamide (b) (Copyright Univ. Innsbruck).
We investigated the approach of selective non-destructive dissolution of elastane from its blends with PET, PA and cotton fibres. A combination of two solvents, which are both widely used in other industrial processes, was found to be very effective for this purpose. One solvent, which is the larger component, is fully biodegradable and sourced from non-edible agriculture waste. The second solvent is a high boiling, non-toxic polar solvent. With this solvent blend, the elastane can be fully dissolved in minutes at room temperature, while the other fibre types component remain intact and available for further processing. The elastane dissolved in the blend, can be recovered by simply evaporating the low boiling solvent at mild temperatures (60 °C) under reduced pressure.
Initial laboratory scale results
Initial investigation on pre-consumer textile waste as models demonstrates that this solvent blend treatment can successfully separate elastane from different fibre materials. The dissolution time strongly depends on the accessibility of the elastane for the solvent. Our initial investigations show that full dissolution is usually reached in less than 1 hour. Figure 2 shows the elastane core in a PET elastomeric garment being dissolved in the solvent blend.
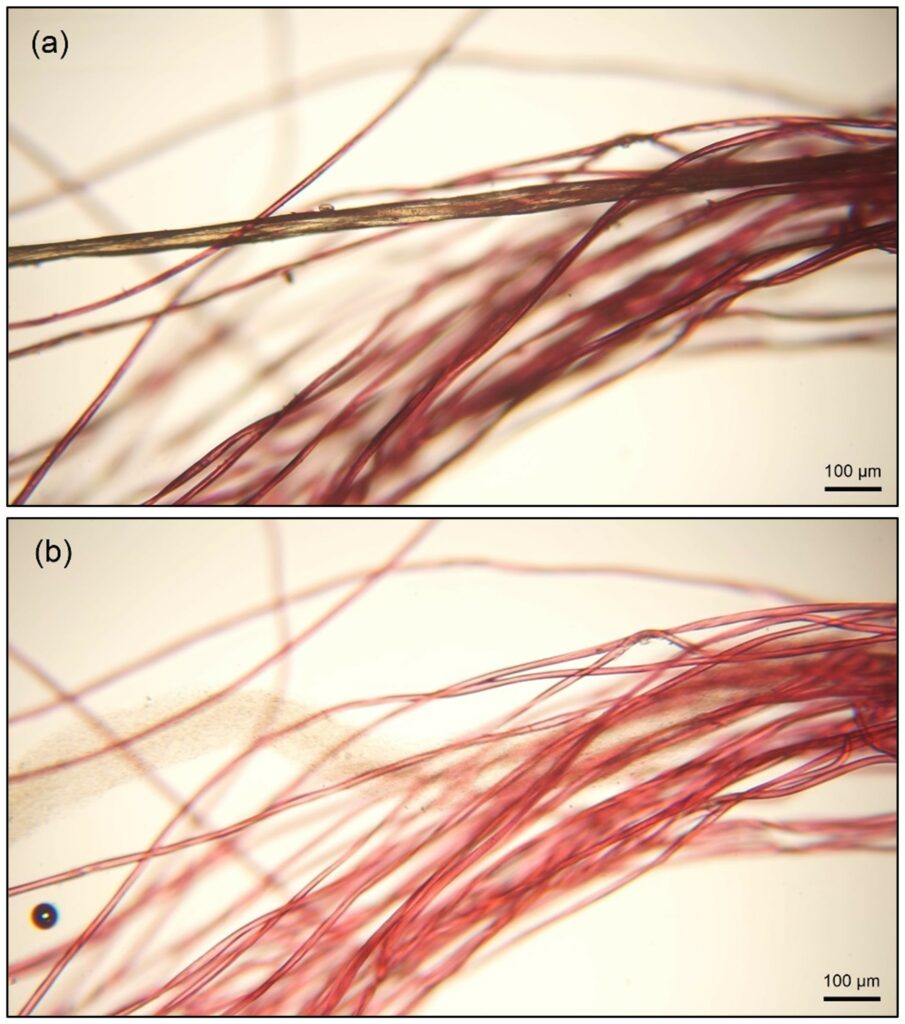
Figure 2. Dissolution of elastane in a PET elastomeric garment: (a) before and (b) five seconds after adding a small amount of solvent (Copyright Univ. Innsbruck).
The potential of this separation technology is being tested in different textile fibre mixture systems such as PET/elastane, polyamide/elastane and cotton/elastane. Figure 3 demonstrates the selective dissolution and subsequent precipitation of elastane.
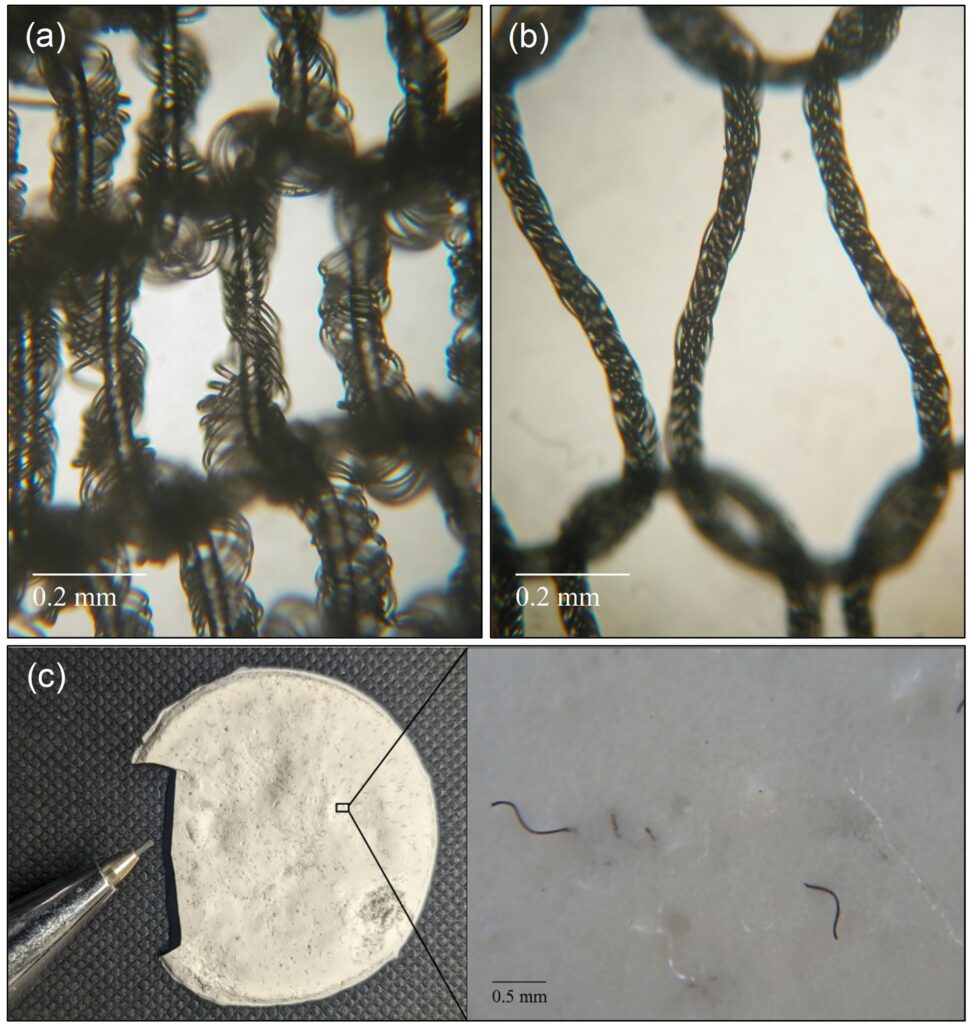
Figure 3. Selective separation of elastane from nylon stockings: (a) before and (b) after treatment, and precipitated elastane with undissolved PA fibres (c) (Copyright Univ. Innsbruck).
For this described process, a patent has been filed ** and will be further investigated to optimise the dissolution efficiency of the system and for larger scale applications. Regarding efficiency, environmental and health concerns, this method may be a preferable way for elastane separation.
* in some cases the proportions may be larger, up to about 30%
** T. Bechtold, M. Cordin, T. Pham, L. Vonbrül, Method For Separating Polyurethane From a Textile, EP22198684.7; 29.09.2022
Project coordination (Story)
Univ.-Prof. Dr. Tung Pham
Research Institute of Textile Chemistry
and Textile Physics
Universität Innsbruck
T +43 5572 28533
textilchemie@uibk.ac.at
www.uibk.ac.at/textilchemie
COMET-Project TCCV2
Research Institute of Textile Chemistry
and Textile Physics,
Universität Innsbruck
Hoechsterstrasse 73, A-6850 Dornbirn
T +43 5572 28533
textilchemie@uibk.ac.at
www.tccv.eu
This success story was provided by the consortium leader and by the mentioned project partners for the purpose of being published on the FFG website. TCCV is a COMET Project within the COMET – Competence Centers for Excellent Technologies Programme and funded by BMK, BMDW and the state of Vorarlberg. The COMET Programme is managed by FFG. Further information on COMET: www.ffg.at/comet

Austrian Research Promotion Agency
Sensengasse 1, A-1090 Vienna
P +43(0)57755-0
office@ffg.at
www.ffg.at